Competitive adsorption characteristics and diffusion process of CO2−CH4 on mineral surface: A case study of the 2nd Section of Shanxi Formationin Yan 'an Gas Field
-
摘要:研究目的
CO2−EGR技术能提高天然气采收率,同时将CO2永久封存于地下,助力实现碳中和目标。CO2−CH4在纳米孔隙中竞争吸附和扩散是增采和封存的关键机理。
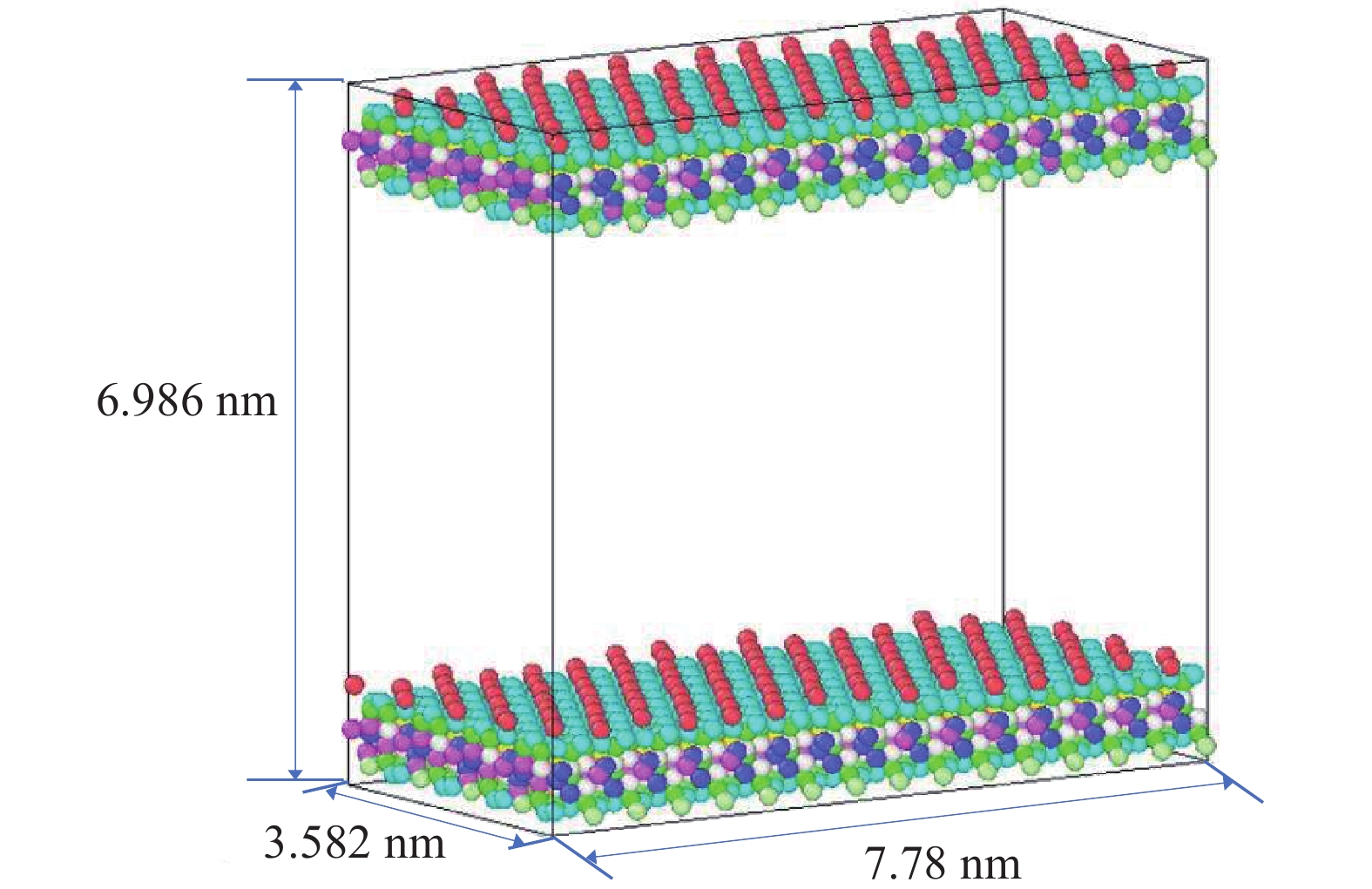
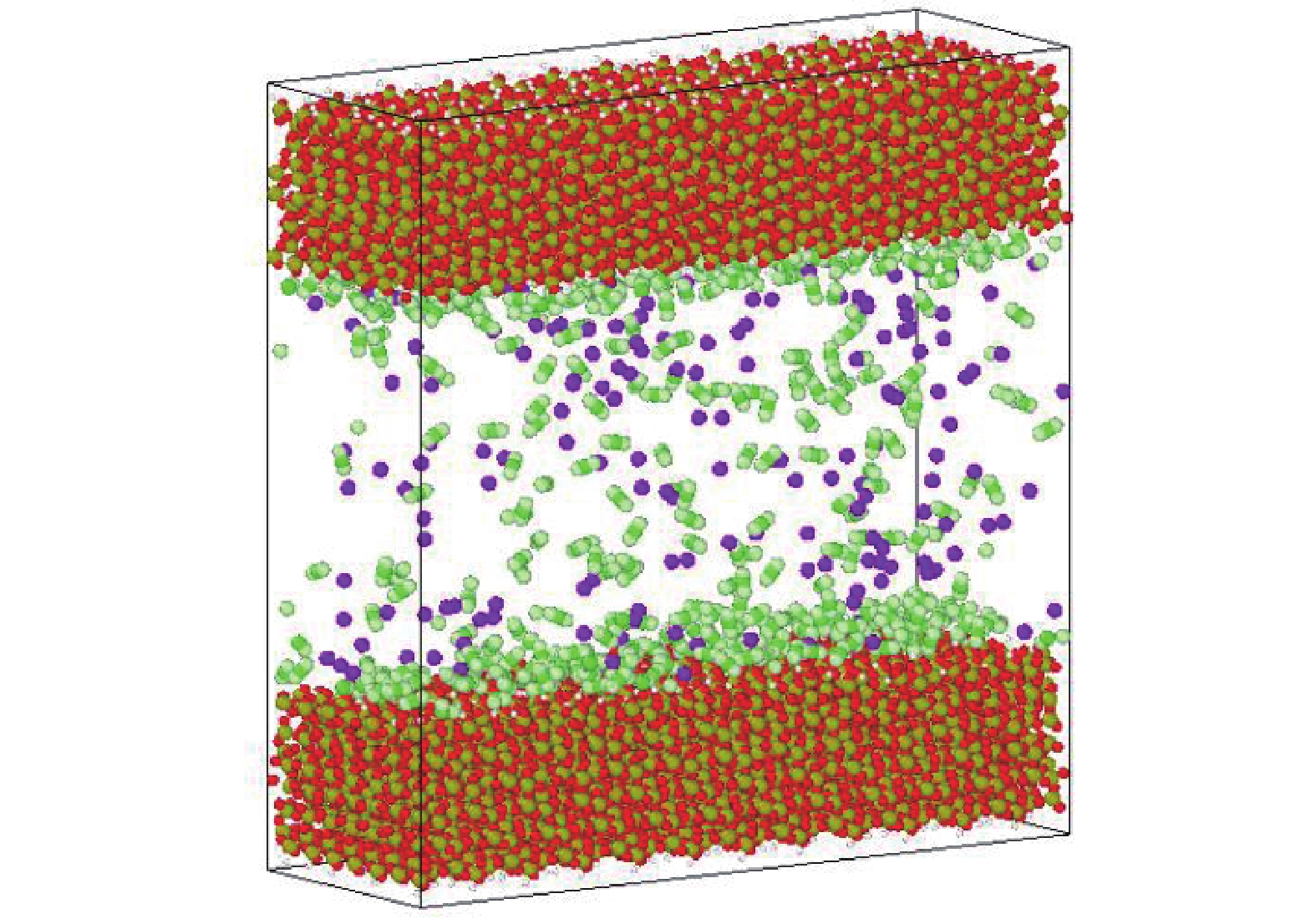
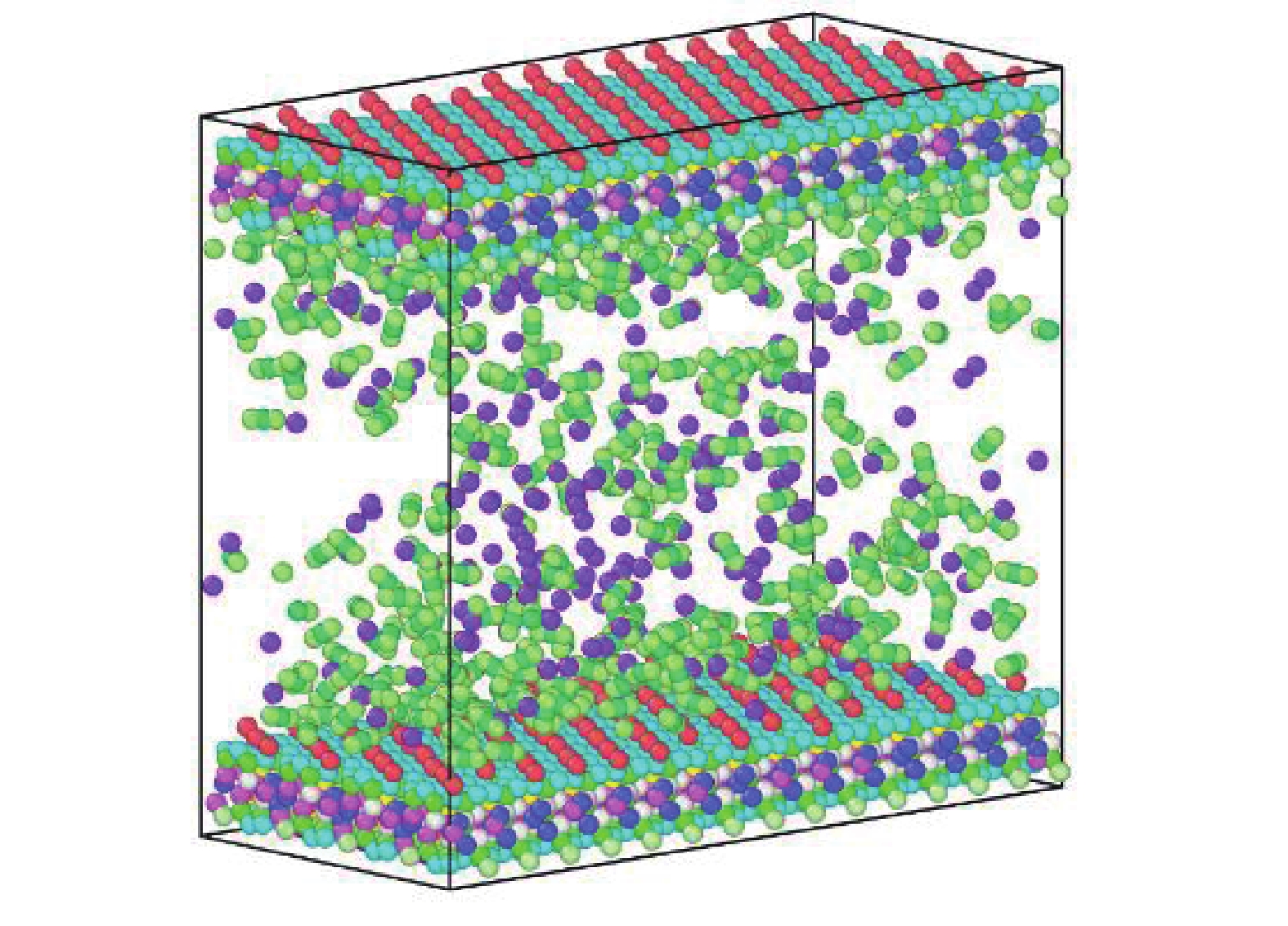
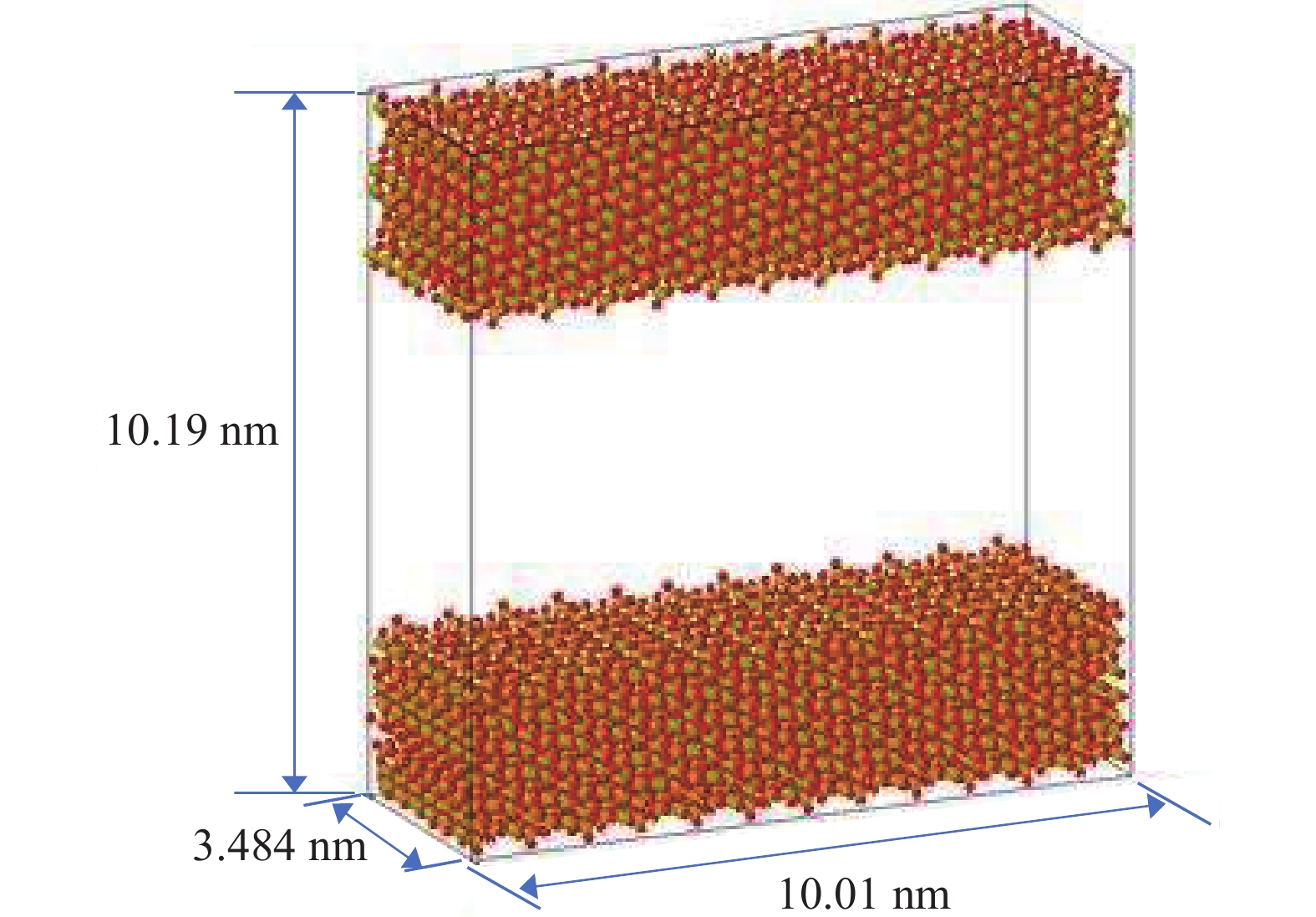
研究方法本文以鄂尔多斯盆地延安气田山2储层为靶区,利用分子动力学(MD)和巨正则系统蒙特卡罗(GCMC)方法建立模型,研究储层温压条件下CO2−CH4混合气体在关键矿物(石英和伊利石)纳米基质孔隙中的竞争吸附规律,分析CO2自扩散系数与温压关系。
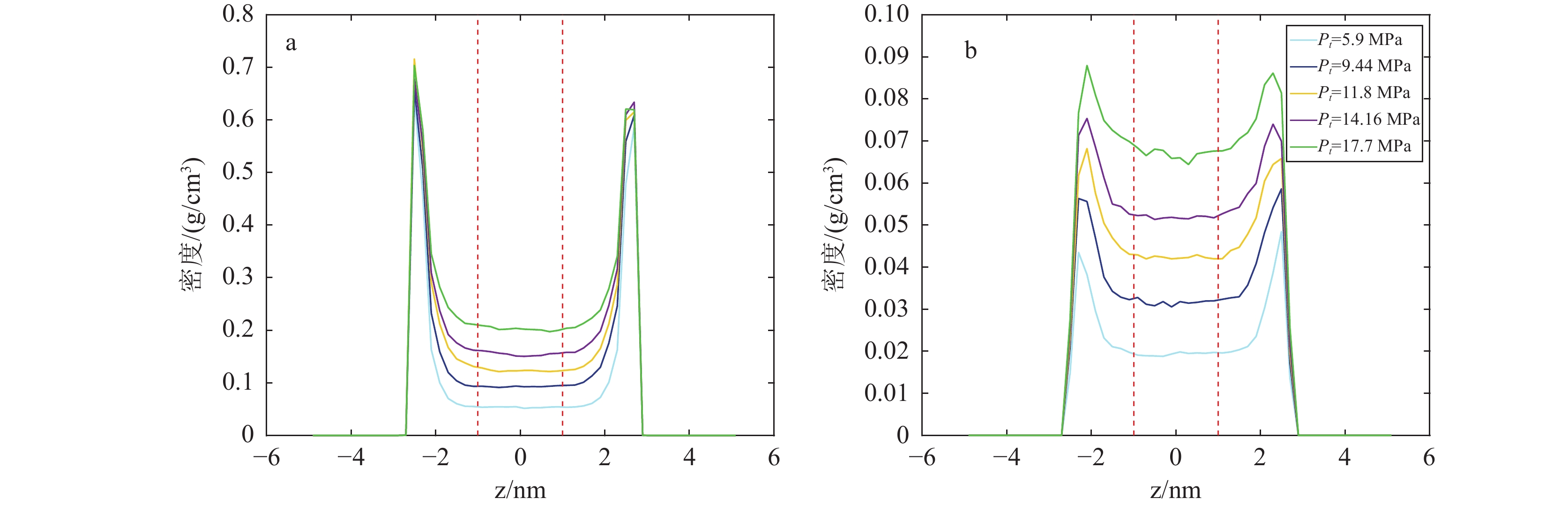
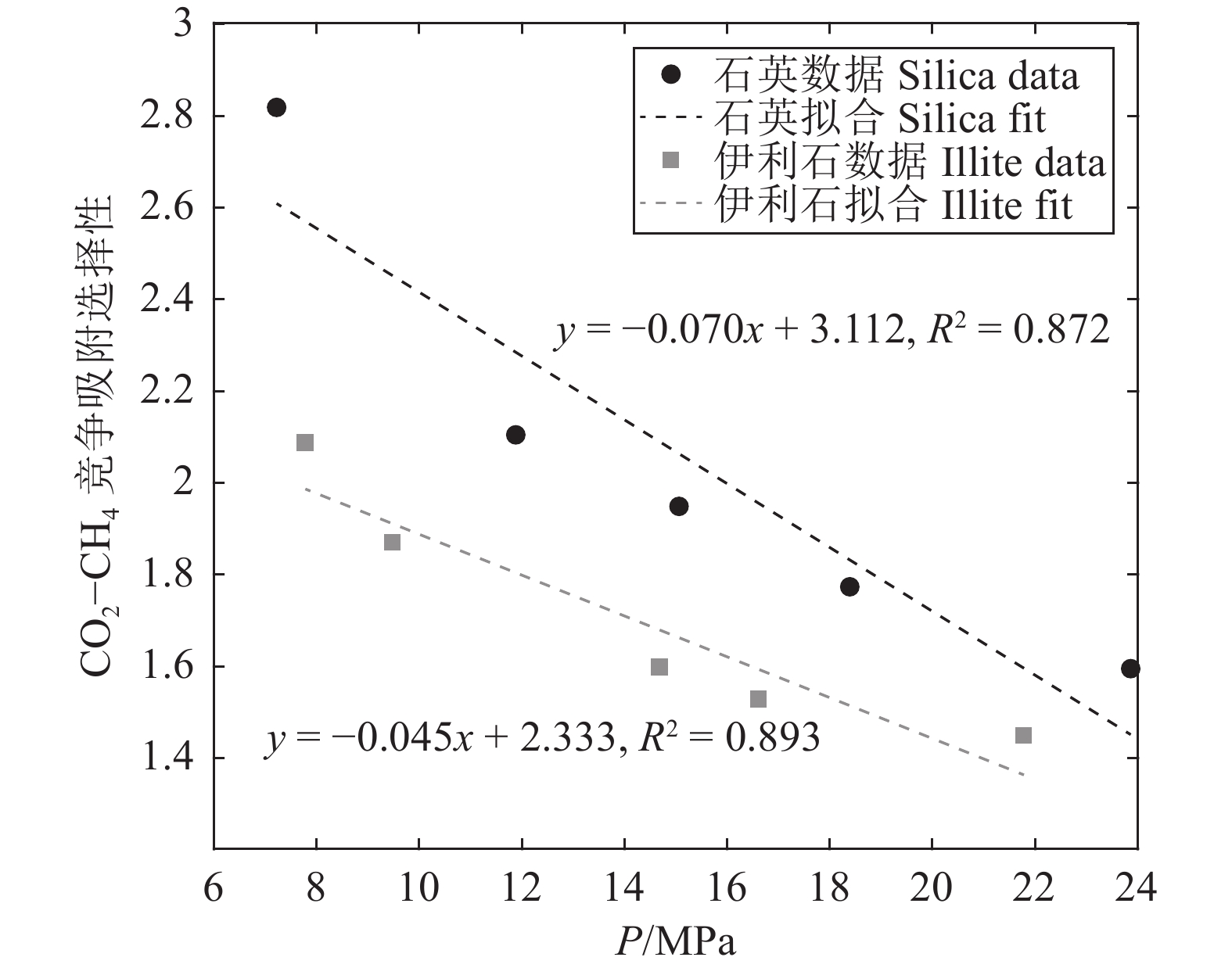
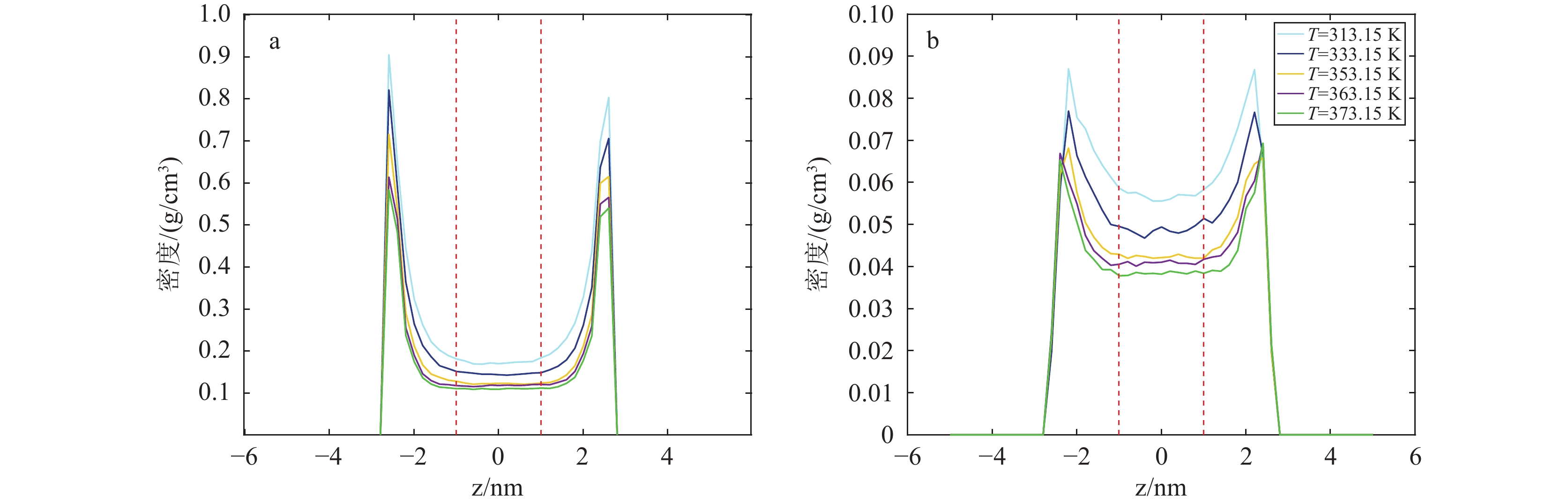
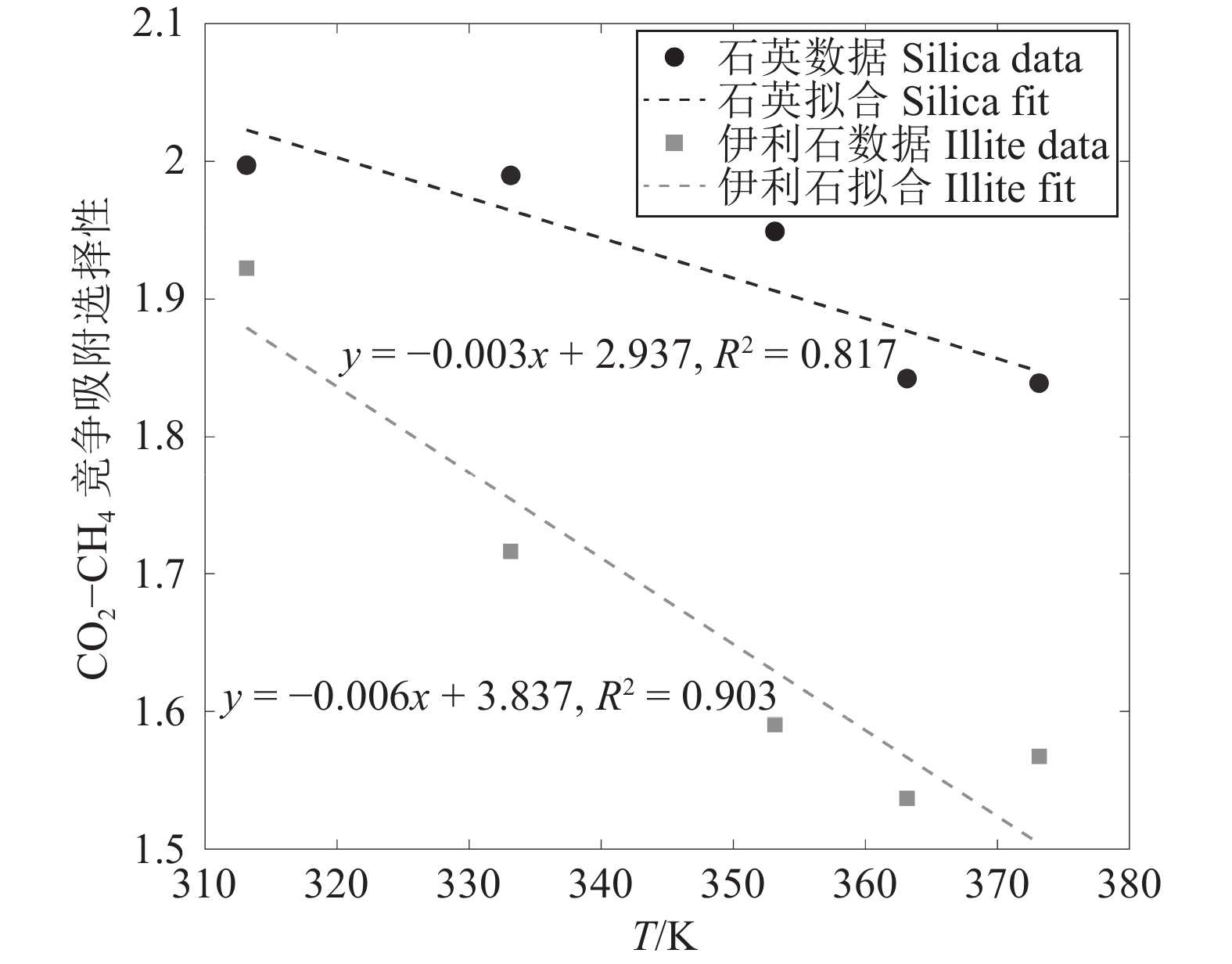
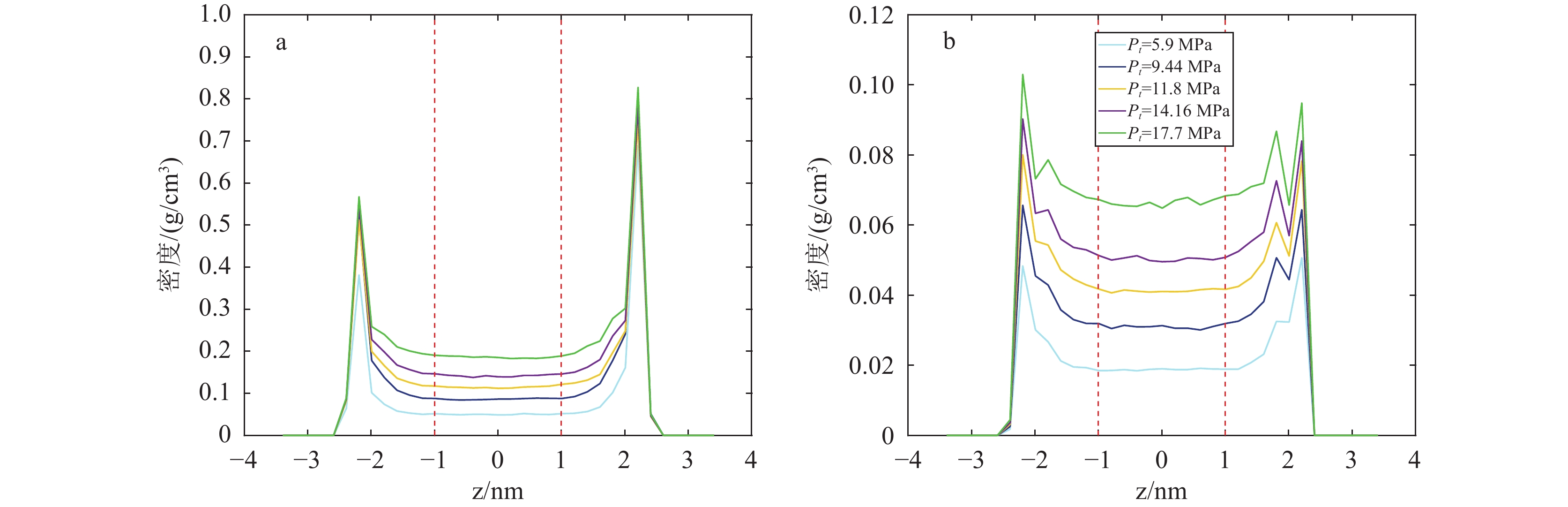
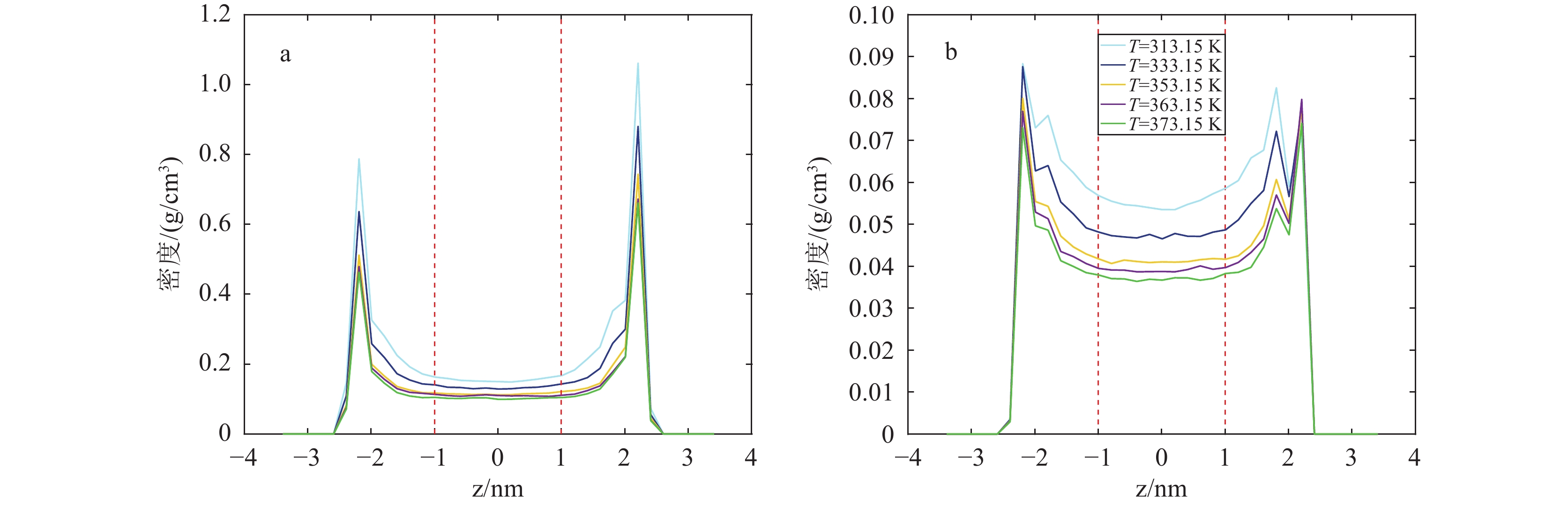
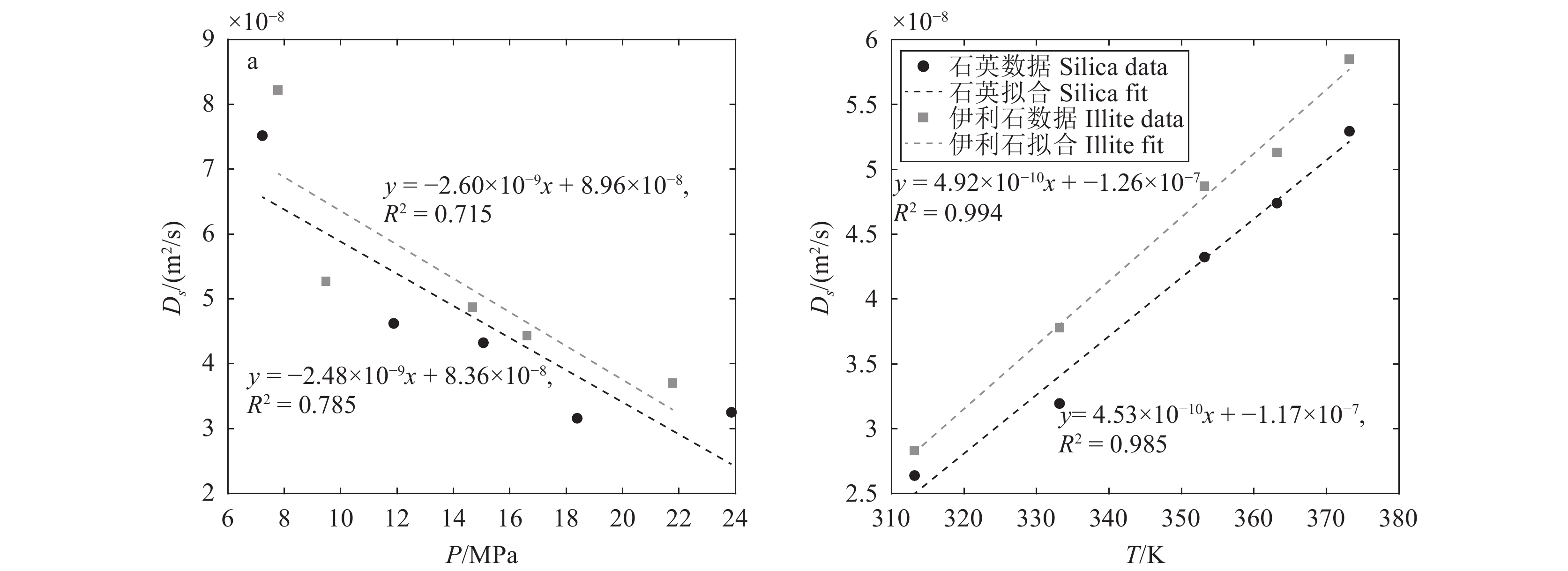
研究结果(1)在等温(353.15 K)变压(5.9~17.7 MPa)和等压(11.8 MPa)变温(313.15~373.15 K)条件下,石英和伊利石对CO2吸附能力大于CH4,CO2−CH4在石英孔隙中的竞争吸附选择性大于伊利石孔隙;(2)等温(353.15 K)变压(5.9~17.7 MPa)和等压(11.8 MPa)变温(313.15~373.15 K)条件下, CO2−CH4在石英和伊利石孔隙中的竞争吸附选择性分别随压力、温度的增加而降低;(3)低压(5.9 MPa)高温(373.15 K)条件下,CO2在CO2−CH4−石英和CO2−CH4−伊利石系统的流动和扩散效率更高。
结论石英和伊利石对CO2吸附量更高,置换CH4能力更高,CO2封存效果更好。
创新点:阐明了纳米孔隙中CO2−CH4的竞争吸附和扩散规律;指出了延安气田山西组2段储层条件下CO2自扩散特性。
Abstract:This paper is the result of environmental geological survey engineering.
ObjectiveThe CO2−Enhanced Gas Recovery (CO2−EGR) technology significantly augments natural gas extraction efficiency while concurrently facilitating the permanent subsurface sequestration of CO2. This dual benefit substantially aids in achieving carbon neutrality goals. The mechanisms pivotal to enhanced recovery and storage include the competitive adsorption and diffusion of CO2−CH4 within nanopores.
MethodsThis study focuses on the 2ndsection of Shanxi formation in the Yan'an Gas Field located in the Ordos Basin. Using molecular dynamics (MD) and grand canonical Monte Carlo (GCMC) methods, a model was established toinvestigate the competitive adsorption behaviors of CO2−CH4 mixed gases in the nanoporous matrices of key minerals, specifically quartz and illite, under reservoir−specific temperature and pressure conditions. Additionally, the study analyzes the correlation between the self−diffusion coefficient of CO2 and the variabilities in temperature and pressure.
ResultsThe study yields several findings: (1) At an isothermal condition of 353.15 K and varying pressures from 5.9 to 17.7 MPa, both quartz and illite exhibit heightened adsorptive capacities for CO2 in comparison to CH4. Furthermore, the competitive adsorption selectivity for CO2−CH4 is found to be greater in quartz pores than in illite pores. (2) Under similar isothermal conditions and at a constant pressure of 11.8 MPa with temperatures ranging from 313.15 K to 373.15 K, the competitive adsorption selectivity for CO2−CH4 in both quartz and illite pores is observed to diminish with increasing pressure and temperature. (3) Under conditions of low pressure (5.9 MPa) and high temperature (373.15 K), there is an enhancement in the mobility and diffusion efficiency of CO2 within both CO2−CH4−quartz and CO2−CH4−illite systems.
ConclusionQuartz and illite have higher CO2 adsorption capacity, greaterCH4displacement capacity, and better CO2 storage effect.
Highlights:The competitive adsorption and diffusion behavior of CO2−CH4 in nanopores is clarified, and the self-diffusion characteristics of CO2 under the reservoir conditions of the 2nd Section of the Shanxi Formation in the Yan’an Gas Field is identified.
-
-
表 1 不同压力下CO2−CH4等摩尔化学势及计算压力和摩尔比(温度T = 353.15 K)
Table 1 Equimolar chemical potential, calculated pressure, and molar ratio of CO2−CH4 at different pressures (temperature T = 353.15 K)
目标压力/
MPaCO2化学势/
(Kcal/mol)CH4化学势/
(Kcal/mol)计算混合气体压力/
MPaCO2−CH4
摩尔比5.90 −9.4468 −8.2991 5.93 1.01 9.44 −9.1792 −8.0029 9.27 1.01 11.80 −9.0491 −7.8357 11.82 1.00 14.16 −8.9538 −7.716 14.13 1.02 17.70 −8.8396 −7.5482 18.07 1.01 表 2 不同温度下CO2−CH4等摩尔化学势及计算压力和摩尔比(压力P=11.8 MPa)
Table 2 Equimolar chemical potential, calculated pressure, and molar ratio of CO2−CH4 at different temperatures (pressure P = 11.8 MPa)
温度/
KCO2化学势/
(Kcal/mol)CH4化学势/
(Kcal/mol)计算混合
气体压力/MPaCO2−CH4
摩尔比313.15 −6.7985 −7.9549 11.54 1.01 333.15 −7.3047 −8.4817 11.93 1.00 353.15 −9.0491 −7.8357 11.82 1.00 363.15 −8.0965 −9.3324 11.85 0.99 373.15 −8.3638 −9.6213 11.84 0.99 表 3 不同目标压力下混合气体压力在无孔隙(仅CO2和CH4)和孔隙中的对比
Table 3 Comparison of mixed gas pressures in no pores (only CO2 and CH4) and pores under different target pressures
目标压力/
MPa混合气体压力−
无孔隙/MPa混合气体压力−
石英孔隙/MPa混合气体压力−
伊利石孔隙/MPa5.90 5.93 7.22 7.78 9.44 9.27 11.88 9.48 11.80 11.82 15.06 14.68 14.16 14.13 18.39 16.61 17.70 18.07 23.86 21.78 表 4 不同温度下混合气体压力在无孔隙(仅CO2和CH4)和孔隙中的对比(目标压力为11.8 MPa)
Table 4 Comparison of mixed gas pressure in no pores (only CO2 and CO2) and pores at different temperatures (target pressure is 11.8 MPa)
温度
(K)混合气体压力−
无孔隙 (MPa)混合气体压力−
石英孔隙 (MPa)混合气体压力−
伊利石孔隙 (MPa)313.15 11.54 16.04 15.72 333.15 11.93 15.68 15.24 353.15 11.82 15.11 14.78 363.15 11.85 15.69 15.17 373.15 11.84 15.00 13.62 -
[1] Cygan R T, Liang J J, Kalinichev A G. 2004. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field[J]. The Journal of Physical Chemistry B, 108: 1255−1266.
[2] Dong Dazhong, Zou Caineng, Li Jianzhong, Wang Shejiao, Li Xinjing, WangYuman, Li Denghua, Huang Jinliang. 2011. Shale gas resource potential and exploration and development prospect[J]. Geological Bulletin of China, 31(2): 324−336 (in Chinese with English abstract).
[3] Emami F S, Puddu V, Berry R J, Varshney V, Patwardhan S V, Perry C C, Heinz H. 2014. Force field and a surface model database for silica to simulate interfacial properties in atomic resolution[J]. Chemistry of Materials, 26: 2647−2658.
[4] Hamza A, Hussein I A, Al–Marri M J, Mahmoud M, Aparicio S. 2021b. CO2 enhanced gas recovery and sequestration in depleted gas reservoirs: A review[J]. Journal of Petroleum Science and Engineering, 196: 107685.
[5] Hamza A, Hussein I A, Al–Marri M J, Mahmoud M, Shawabkeh R. 2021a. Impact of clays on CO2 adsorption and enhanced gas recovery in sandstone reservoirs[J]. International Journal of Greenhouse Gas Control, 106: 103286.
[6] Hao Y, Yuan L, Li P, Zhao W, Li D, Lu D. 2018. Molecular simulations of methane adsorption behavior in illite nanopores considering basal and edge surfaces[J]. Energy & Fuels, 32: 4783−96.
[7] Harris J G, Yung K H. 1995. Carbon dioxide's liquid–vapor coexistence curve and critical properties as predicted by a simple molecular model[J]. The Journal of Physical Chemistry, 99: 12021−12024.
[8] Holmboe M. 2019. atom: A MATLAB package for manipulation of molecular systems[J]. Clays and Clay minerals, 67: 419−426.
[9] Humphrey W, Dalke A, Schulten K. 1996. VMD: Visual molecular dynamics[J]. Journal of Molecular Graphics, 14: 33−38.
[10] Jing Shasha. 2015. Molecular Simulation of CO2/CH4 Mass Transfer Process in Sandstone Micropores[D]. Chengdu: Southwest Petroleum University, 55 (in Chinese with English abstract).
[11] Ju Huijiao, Sun Wei, Yang Xipu, Han Zongyuan. 2011. Reservoir characteristics and main controlling factors of Shan–2 member, Yan 'an Area, Ordos Basin[J]. Fault–block Oil and Gas Field, 18(2): 142−145,157 (in Chinese with English abstract).
[12] Le T, Striolo A, Cole D R. 2015. CO2–C4H10 mixtures simulated in silica slit pores: Relation between structure and dynamics[J]. The Journal of Physical Chemistry C, 119: 15274−15284.
[13] Lei Qun, Wang Hongyan, Zhao Qun, Liu Dexun. 2008. Current situation and suggestions on exploration and development of unconventional oil and gas resources at home and abroad[J]. Natural Gas Industry, 28(12): 7−10 (in Chinese with English abstract).
[14] Lv Fangtao, Ning Zhengfu, Mu Zhongqi, Jia Zejiang, Liu Bei. 2023. Molecular simulation of methane flow in rough quartz nanopores[J]. Journal of Northeast Petroleum University, 47(5): 82−91 (in Chinese with English abstract).
[15] Oldenburg C M, Pruess K, Benson S M. 2001. Process modeling of CO2 Injection into natural gas reservoirs for carbon sequestration and enhanced gas recovery[J]. Energy & Fuels, 15: 293−298.
[16] Plimpton S. 1995. Fast parallel algorithms for short–range molecular dynamics[J]. Journal of Computational Physics, 117: 1−19.
[17] Potoff J J, Siepmann J I. 2001. Vapor–liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen[J]. AIChE Journal, 47: 1676−1682.
[18] Shi L Z, Wang Z Z, Xing Z T, Meng S, Guo, S, Wu S M, Luo L Y. 2023. Geological characteristics of unconventional tight oil reservoir: A case study of Upper Cretaceous Qingshankou Formation, northern Songliao Basin, NE China[J]. China Geology, 6: 1−12.
[19] Song Zhengping, Zhang Bin, Kang Tianhe. 2018. Molecular simulation of competitive adsorption of CO2/CH4 in kaolinite based on adsorption site theory[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 37(4): 724–730 (in Chinese with English abstract).
[20] Sun H Y, Zhao H, Qi N, Qi X Q, Zhang K, Sun W C, Li Y. 2016. Mechanistic insight into the displacement of CH4 by CO2 in calcite slit–nanopores: the effect of competitive adsorption[J]. RSC Advances, 6: 104456−104462. doi: 10.1039/C6RA23456A
[21] Sun Haoyang. 2014. Study on Micro–mechanism of Carbon Dioxide Enhanced Shale Recovery[D]. Jinan: Shandong University, 1–103(in Chinese with English abstract).
[22] Sun Ying. 2021. Mechanism of CO2 Enhanced Oil recovery in shale Gas reservoirs based on competitive adsorption[D]. Dongying: China University of Petroleum (East China), 1–116 (in Chinese with English abstract).
[23] Wang Xiangzeng, Qiao Xiangyang, Mi Naizhe, Wang Ruogu. 2018. Beneficial development supporting technology of low permeability tight sandstone gas reservoir in Yan 'an Gas field[J]. Natural Gas industry, 38(11): 43−51 (in Chinese with English abstract).
[24] Wang Xiangzeng. 2016. Progress of unconventional gas exploration and development in Yanchang Petroleum Group[J]. Acta Petrolei Sinica, 37(1): 137−144 (in Chinese with English abstract).
[25] Watts R. 1996. Objectives of the U. S. DOE's research[J]. The Leading Edge, 15: 906−906.
[26] Yang Hu, Wang Jianmin. 2015. Study on tight sandstone reservoir and micro–pore throat characteristics of Shanxi Formation in Yanchang Gas field[J]. Journal of Xi 'an University of Science and Technology, 35(6): 755−762 (in Chinese with English abstract).
[27] Zhang Minghang, Guo Ping, Jiang Wei, Chen Hong. 2016. Study on adsorption characteristics of CO2/CH4 in illite[J]. World Science and Technology Research and Development, 38(5): 950−954 (in Chinese with English abstract).
[28] Zhang Xinmin, Zheng Hongqing, Li Gangyao. 2008. Research on fracturing technology of low permeability tight sandstone gas reservoir in Junggar Basin[J]. Xinjiang Oil and Gas, 4(S1): 69−72 (in Chinese with English abstract).
[29] 董大忠, 邹才能, 李建忠, 王社教, 李新景, 王玉满, 李登华, 黄金亮 2011. 页岩气资源潜力与勘探开发前景[J]. 地质通报, 31(2): 324–336. [30] 景莎莎. 2015. 砂岩微孔隙中CO2/CH4传质过程的分子模拟研究[D]. 成都: 西南石油大学, 1–55. [31] 琚惠姣, 孙卫, 杨希濮, 韩宗元 2011. 鄂尔多斯盆地延安地区山2段储层特征及其主控因素[J]. 断块油气田, 18(2): 142–145, 157. [32] 雷群, 王红岩, 赵群, 刘德勋. 2008. 国内外非常规油气资源勘探开发现状及建议[J]. 天然气工业, 28(12): 7−10. doi: 10.3787/j.issn.1000-0976.2008.12.003 [33] 吕方涛, 宁正福, 穆中奇, 贾泽江, 刘蓓. 2023. 粗糙石英纳米孔隙甲烷流动分子模拟[J]. 东北石油大学学报, 47(5): 82−91. doi: 10.3969/j.issn.2095-4107.2023.05.007 [34] 宋正平, 张彬, 康天合 2018. 基于吸附位理论的CO2/CH4在高岭石中竞争吸附的分子模拟[J]. 矿物岩石地球化学通报, 37(4): 724–730. [35] 孙浩洋. 2014. 二氧化碳提高页岩采收率的微观机制研究[D]. 济南: 山东大学, 1–103. [36] 孙仁远, 张云飞, 范坤坤, 史永宏, 杨世凯. 2015. 岩中黏土矿物吸附特性分子模拟[J]. 化工学报, 66(6): 2118−2122. [37] 孙莹. 2021. 基于竞争吸附的页岩气藏CO2提高采收率机理研究[D]. 东营: 中国石油大学(华东), 1–116. [38] 王香增. 2016. 延长石油集团非常规天然气勘探开发进展[J]. 石油学报, 37(1): 137−144. [39] 王香增, 乔向阳, 米乃哲, 王若谷. 2018. 延安气田低渗透致密砂岩气藏效益开发配套技术[J]. 天然气工业, 38(11): 43−51. doi: 10.3787/j.issn.1000-0976.2018.11.005 [40] 杨虎, 王建民 2015. 延长气田山西组致密砂岩储层及微观孔喉特征研究[J]. 西安科技大学学报, 35(6): 755–762. [41] 张明航, 郭平, 蒋炜, 陈红. 2016. CO2/CH4在伊利石中的吸附特性研究[J]. 世界科技研究与发展, 38(5): 950−954. [42] 张新民, 郑洪庆, 李纲要 2008. 准噶尔盆地低渗致密砂岩气藏压裂工艺技术研究[J]. 新疆石油天然气, 4(S1): 69–72.



 下载:
下载: